NANOBODY® Technology Platform

These tiny, modular antibodies are ushering in a new era in therapeutics at Sanofi. Here we explain how they work and why they are generating so much excitement.
The serendipitous discovery in 1989 of a special kind of antibody in llamas and other camelid species sparked decades of innovation. It led biotech company Ablynx, now part of Sanofi, to create NANOBODY® technology,1 a revolutionary platform that is enabling our researchers to create new therapeutics and refine existing treatments.
What are NANOBODY® molecules?
NANOBODY molecules are a type of miniature, engineered antibody. Antibodies have both "heavy" and "light" chains of amino acids (peptides). Human antibodies have both types, but llamas, alpacas, and other species make antibodies that have only "heavy-chain" peptides. NANOBODY molecules are derived from these "heavy-chain-only" peptides, and are around a tenth the size of conventional antibodies.2,3

Human antibodies have both heavy and light peptide chains, (1) while camelids can also generate heavy-chain-only antibodies (2). NANOBODY® antibodies are based on a fragment of one domain of a heavy-chain antibody
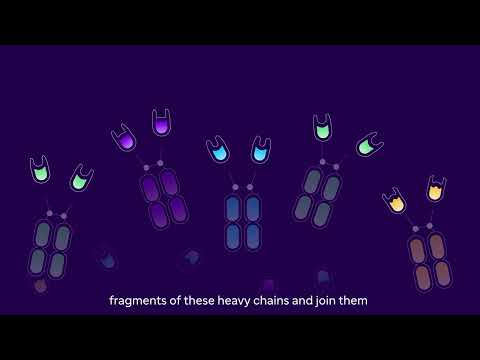
Most antibodies can bind to just one target. But by connecting antibody fragments, like beads on a string, our teams can create new compounds (called "multivalent" NANOBODY molecules) that bind to many different targets at once.1 For example, a single therapeutic NANOBODY molecule might create a bridge between a tumor cell and an immune cell by attaching to several sites on a tumor and an immune cell. This could help the body's immune system fight cancer.

The basic building block of a therapeutic NANOBODY molecule is a fragment from one domain of a heavy-chain antibody (left). Fragments from different heavy-chain antibodies can be strung together (right)

Each building block of the molecule can bind tightly to a specific protein, for example on the surface of a tumor (right) or immune cell (left)
Why does it matter?
NANOBODY molecules have become an essential tool in drug discovery. They are helping our scientists design new therapeutics, and develop medicines that could one day replace complex treatment regimens with single, multi-action medicines. Combined with Sanofi's large-scale manufacturing capacity, NANOBODY molecules and other cutting-edge technologies are empowering our teams develop the medicines of tomorrow.


Intellectual Property
Sanofi Ablynx has a patent portfolio of more than 500 pending and granted patents spanning discovery, generation, optimization, formatting, manufacture, administration, formulation and clinical use of NANOBODY molecules, as well as covering its clinical development programs and product candidates. Sanofi’s affiliate Ablynx N.V. is the holder worldwide of the NANOBODY® trademark.
References
- NANOBODY is a registered trademark of Ablynx N.V.
- Leslie M (2018) Mini-antibodies discovered in sharks and camels could lead to drugs for cancer and other diseases. Science Magazine; doi:10.1126/science.aau1288
- Jovčevska I, Muyldermans S (2020) BioDrugs 34:11–26.
- Ingram JR, Schmidt FI, Ploegh HL (2018) Ann Rev Immonol 36:695–715. See also Hamers-Casterman C, et al. (1993) Nature 363:446-8; doi: 10.1038/363446a0


